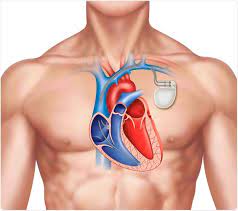
 “The FDA has announced that Boston Scientific is recalling 48,000 INGENIO pacemakers and cardiac resynchronization therapy pacemakers (CRT-Ps). The recall is due to potential issues when the devices transition to safety mode….”
“The FDA has announced that Boston Scientific is recalling 48,000 INGENIO pacemakers and cardiac resynchronization therapy pacemakers (CRT-Ps). The recall is due to potential issues when the devices transition to safety mode….”
To read the full article, please click here.
(Shared from Cardiovascular Business, August 6, 2021)









